Catalysis
Facilitating the Breaking and Making of Bonds
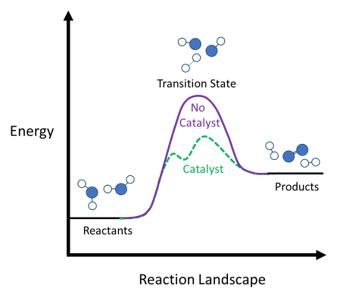
Bonds between atoms are broken and re-formed when enough energy is provided. This phenomenon is called a chemical reaction and is the basis of countless occurrences ranging from the marvel of life to the production of fuels to the cooking of food. Chemical reactions start with reactants which, when provided with enough energy, breaks and makes bonds via a dynamic event called the transition state, before eventually forming product.
When desired chemical reactions are too slow, tampering with the transition state by means of changing the rate or type of interactions between reactants and its environment can lead to faster reaction rates. Several methods are changing reactant pressures, introducing more energy into the system, or introducing a third species to bind with reactants in such a way that the chemical transformation traverses a new more facile transition state route. This third species is called catalyst. Catalysts are not consumed during the reaction and can be re-used – at least in theory – indefinitely.
Our Approach
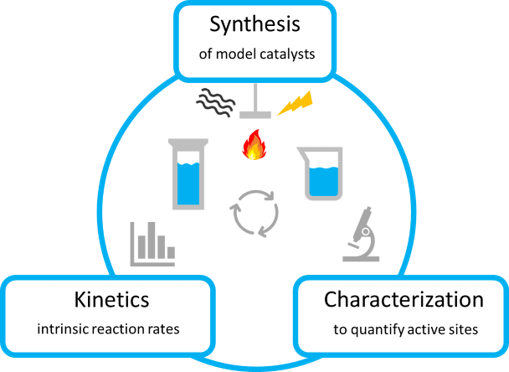
Our research group pieces together how catalytic active sites and its environment interact with reactants to form products – how atoms come together, how bonds break and form, and how other influences from the environment influences this process. Our approach is three-pronged: we (1) synthesize and prepare model materials and elucidate atomic details using (2) careful kinetic rate measurements and (3) other characterization methods such as spectroscopy and microscopy, ideally in operando. An understanding of phenomenon at the atomic level are pieced together from this evidence.
Research directions will be motivated by data-science driven approaches which integrates big data and data-science into the traditional research model. Additionally, collaborations with other groups whose expertise deviates from our own (e.g. computation, synchrotron) helps paint a more cohesive mosasic of atomic phenomenon as we push the forward toward the unknown at the forefront of science.